Condensation, often manifested as water droplets forming on cold surfaces in humid environments, poses a significant risk to product quality and longevity. This phenomenon is especially prevalent during shipping and storage, where temperature fluctuations are common. Any resulting condensation will accelerate corrosion, leading to product defects, performance issues, and financial losses.
Contaminants present on product surfaces exacerbate corrosion when combined with condensation to form an electrolytic solution. The rapid onset of corrosion can result in visible damage upon product unpacking, rendering products unusable or non-compliant with warranty terms. Even when corrosion is not yet visible to the eye, product failure is accelerated due to corrosion.
To mitigate the risks associated with condensation and corrosion, several preventive measures can be implemented:
Read More
Topics:
corrosion,
corrosion prevention,
seasonal corrosion,
protective packaging,
contamination
Without barrier protection in place, paper fiber corrugated cartons may cause corrosion on products stored within. In fact, even a product stored for just four weeks in a corrugated box that meets the archival standards established by the U.S. National Archives and is at the upper limits of the sulfur level specified, would see corrosive sulfur potential exposure equivalent of greater than 20 years of natural exposure. Four weeks is not a lot of time for shipping and storage, especially if that carton is shipped overseas and/or into humid, harsh environments where air pollution is prevalent.
Read More
Topics:
corrosion,
barrier packaging,
packaging,
quality assurance
Corrosion is one of the most underestimated and often misunderstood forces humans deal with on a daily basis. A large part of that underestimation is the image in many people's minds of what corrosion is. We tend to think and talk about corrosion similar to erosion: it's a geological time-scale force with which humans not only needn't engage but indeed shouldn't even concern ourselves, as it would be a futile waste of time and energy. Such a submissive attitude toward the natural forces may serve as a satisfactory spiritual practice, but to the discerning member of any competitive economic system, it is simply untenable. While the idea of battling corrosion may then conjure an image of poor Sisyphus and his infinite boulder displacement task, corrosion control can be done effectively and without anguish, as we've seen in our series of posts on the subject. Of course images and possibilities are interesting, but only hard data will tell the real story.
Read More
Topics:
corrosion,
Corrosion Control
"Corrosion Control" generally refers to the implementation of measures to reduce or eliminate corrosion in:
- Metal
- Concrete
- Water
- Sand
- Masonry
- Soil
Corrosion control consists of different monitoring and control techniques used by industries to solve corrosion problems according to their requirements. Such methods are important to avoiding the expense and negative consequences of corrosion.
Read More
Topics:
corrosion,
corrosion controls,
Corrosion Control
The movie, in 3D, was visually stunning and the extra dimension added layers to the CGI of Mars. I was so immersed that I only noticed the 3D at certain heightened moments, one in particular when snow was falling in front of onlookers on earth. I was fully engrossed.
I read reviews of “The Martian” where some people expressed disappointment that the movie was a departure from the book. Considering the volume of twists and turns (along with explanations of why certain things were tough on Mars and how Watney, the main character played by Matt Damon, solved the problems) it would have been impossible to actually fit all of them in with satisfactory explanations or narration.
Read More
Topics:
corrosion,
rust,
Static Intercept

Galvanic corrosion is a type of corrosion which occurs when two different metals are in contact with each other and an electrolyte. Different metals will have different electric potentials when connected in this way. This difference creates an electric current through the electrolyte. In fact, the action of galvanic corrosion is the principle with which batteries are made. Of course this is also the reason batteries have a shelf life. The action of this circuit degrades whichever metal has a lower electric potential. This is described as being less noble, whereas the metal with the higher potential is more noble. The degradation of the less noble metal eventually gets to the point that the circuit is broken by the oxides and salts created by the corrosion. This is the reason not only for a battery’s eventual death, but also for the way it dies, slowly losing electric potential because the anode (lower potential metal or connection) is slowly destroyed by the action of galvanic corrosion.
Read More
Topics:
corrosion,
chloride,
reasons for packaging,
electronics corrosion
I'm very excited about the movie The Martian. Clearly I am not alone; after its opening day it received high audience ratings and near-to-box-office-record receipts. Although that may be because of the self-selected group anticipating to see it on opening day, let me give you a few reasons why you should be excited too.
Read More
Topics:
corrosion,
Intercept Technology,
reasons for packaging,
rust
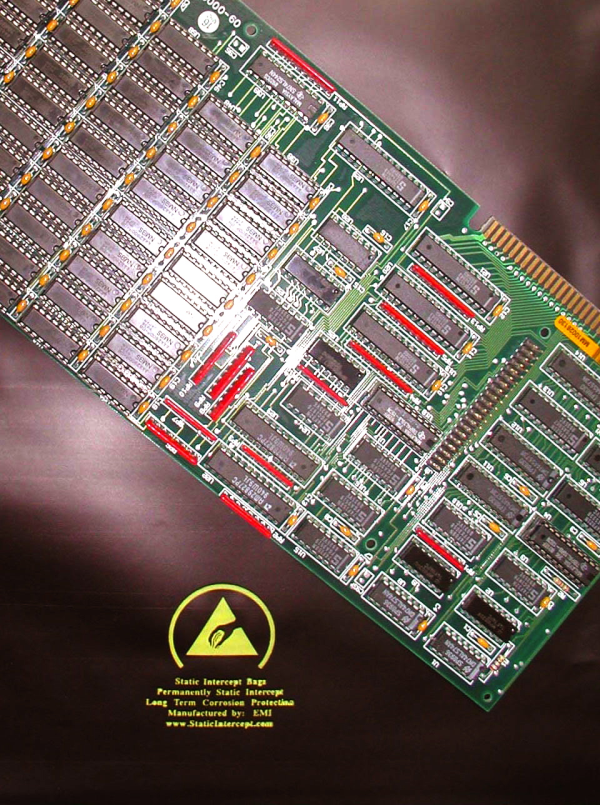
To be clear, the difference between electronics and other electrical systems is that electronics include active components to control the flow of electricity, whereas non-electronic electrical systems use mechanical switches or relays. The development of the vacuum tube (the first active component invented) allowed for the creation of far more complex systems than was possible with prior technology. Then solid-state transistors allowed electronics to shrink to sizes unthinkable before. Certainly at this point it is trivial to say that electronics are ubiquitous in society today and will only continue to become more so in the coming years, all the way up to the singularity, at which point we will become our own technology. As electronics have developed through the years, they have been given increasingly more important tasks. From air traffic control to car computers to medical equipment to missile defense, systems which include electronics control and protect our lives everyday. Thus it is essential that we know how to maintain them, for which we must also know how they degrade.
Read More
Topics:
corrosion,
chloride,
electronics packaging,
aluminum,
electronics corrosion
Chlorine is one of the most common elements found on Earth's crust. The name comes from the Greek word for light green, which is how the gas appears in elemental form. It has 17 protons and two stable isotopes giving it a standard atomic weight of 35.45, which makes chlorine the second lightest halogen. It also has the highest electron affinity of any element making it a very strong oxidizer. This means that chlorine will readily steal electrons from other elements. In fact the vast majority of chlorine found on Earth is in the form of the chloride anion (a chlorine atom which has already stolen an extra electron), which will form ionic compounds with many cations (like metals). It is in this form that humans are most familiar with chlorine, as in ionic compound sodium chloride, which we know of as table salt. Chloride ions are important to many chemical and industrial processes including the making of usable chlorine and sodium hydroxide, and desalination and testing of potable water.
Read More
Topics:
corrosion,
corrosion intercept,
pitting corrosion,
chloride
Liberty Intercept Blog
Condensation and Corrosion Control: Protect Product Integrity
Posted by Joe Spitz on Aug 16, 2024 1:39:48 PM
Condensation, often manifested as water droplets forming on cold surfaces in humid environments, poses a significant risk to product quality and longevity. This phenomenon is especially prevalent during shipping and storage, where temperature fluctuations are common. Any resulting condensation will accelerate corrosion, leading to product defects, performance issues, and financial losses.
Contaminants present on product surfaces exacerbate corrosion when combined with condensation to form an electrolytic solution. The rapid onset of corrosion can result in visible damage upon product unpacking, rendering products unusable or non-compliant with warranty terms. Even when corrosion is not yet visible to the eye, product failure is accelerated due to corrosion.
To mitigate the risks associated with condensation and corrosion, several preventive measures can be implemented:
Read MoreTopics: corrosion, corrosion prevention, seasonal corrosion, protective packaging, contamination
Corrosion via Corrugate
Posted by Joe Spitz on Jan 9, 2020 10:29:19 AM
Without barrier protection in place, paper fiber corrugated cartons may cause corrosion on products stored within. In fact, even a product stored for just four weeks in a corrugated box that meets the archival standards established by the U.S. National Archives and is at the upper limits of the sulfur level specified, would see corrosive sulfur potential exposure equivalent of greater than 20 years of natural exposure. Four weeks is not a lot of time for shipping and storage, especially if that carton is shipped overseas and/or into humid, harsh environments where air pollution is prevalent.
Read MoreTopics: corrosion, barrier packaging, packaging, quality assurance
What is Corrosion?
Posted by Greg Spitz on Nov 28, 2017 4:37:29 PM
Topics: corrosion
The Real Cost of Corrosion
Posted by Greg Spitz on Dec 14, 2016 9:05:02 AM
Corrosion is one of the most underestimated and often misunderstood forces humans deal with on a daily basis. A large part of that underestimation is the image in many people's minds of what corrosion is. We tend to think and talk about corrosion similar to erosion: it's a geological time-scale force with which humans not only needn't engage but indeed shouldn't even concern ourselves, as it would be a futile waste of time and energy. Such a submissive attitude toward the natural forces may serve as a satisfactory spiritual practice, but to the discerning member of any competitive economic system, it is simply untenable. While the idea of battling corrosion may then conjure an image of poor Sisyphus and his infinite boulder displacement task, corrosion control can be done effectively and without anguish, as we've seen in our series of posts on the subject. Of course images and possibilities are interesting, but only hard data will tell the real story.
Read MoreTopics: corrosion, Corrosion Control
Corrosion Control - Let's Break It Down
Posted by Elaine Spitz on Aug 1, 2016 4:16:14 PM
"Corrosion Control" generally refers to the implementation of measures to reduce or eliminate corrosion in:
Corrosion control consists of different monitoring and control techniques used by industries to solve corrosion problems according to their requirements. Such methods are important to avoiding the expense and negative consequences of corrosion.
Read MoreTopics: corrosion, corrosion controls, Corrosion Control
The Martian - Part II - Adventure on the Rust Planet
Posted by Elaine Spitz on Oct 30, 2015 11:37:00 AM
The movie, in 3D, was visually stunning and the extra dimension added layers to the CGI of Mars. I was so immersed that I only noticed the 3D at certain heightened moments, one in particular when snow was falling in front of onlookers on earth. I was fully engrossed.
I read reviews of “The Martian” where some people expressed disappointment that the movie was a departure from the book. Considering the volume of twists and turns (along with explanations of why certain things were tough on Mars and how Watney, the main character played by Matt Damon, solved the problems) it would have been impossible to actually fit all of them in with satisfactory explanations or narration.
Topics: corrosion, rust, Static Intercept
Galvanic Corrosion: It's In Your Electronics
Posted by Greg Spitz on Oct 13, 2015 4:50:00 PM
Galvanic corrosion is a type of corrosion which occurs when two different metals are in contact with each other and an electrolyte. Different metals will have different electric potentials when connected in this way. This difference creates an electric current through the electrolyte. In fact, the action of galvanic corrosion is the principle with which batteries are made. Of course this is also the reason batteries have a shelf life. The action of this circuit degrades whichever metal has a lower electric potential. This is described as being less noble, whereas the metal with the higher potential is more noble. The degradation of the less noble metal eventually gets to the point that the circuit is broken by the oxides and salts created by the corrosion. This is the reason not only for a battery’s eventual death, but also for the way it dies, slowly losing electric potential because the anode (lower potential metal or connection) is slowly destroyed by the action of galvanic corrosion.
Topics: corrosion, chloride, reasons for packaging, electronics corrosion
Adventure on the Rust Planet: The Martian
Posted by Elaine Spitz on Oct 13, 2015 9:07:00 AM
I'm very excited about the movie The Martian. Clearly I am not alone; after its opening day it received high audience ratings and near-to-box-office-record receipts. Although that may be because of the self-selected group anticipating to see it on opening day, let me give you a few reasons why you should be excited too.
Read MoreTopics: corrosion, Intercept Technology, reasons for packaging, rust
Electronics Corrosion
Posted by Greg Spitz on Oct 6, 2015 9:12:00 AM
To be clear, the difference between electronics and other electrical systems is that electronics include active components to control the flow of electricity, whereas non-electronic electrical systems use mechanical switches or relays. The development of the vacuum tube (the first active component invented) allowed for the creation of far more complex systems than was possible with prior technology. Then solid-state transistors allowed electronics to shrink to sizes unthinkable before. Certainly at this point it is trivial to say that electronics are ubiquitous in society today and will only continue to become more so in the coming years, all the way up to the singularity, at which point we will become our own technology. As electronics have developed through the years, they have been given increasingly more important tasks. From air traffic control to car computers to medical equipment to missile defense, systems which include electronics control and protect our lives everyday. Thus it is essential that we know how to maintain them, for which we must also know how they degrade.
Topics: corrosion, chloride, electronics packaging, aluminum, electronics corrosion
Chlorine
Posted by Greg Spitz on Aug 24, 2015 6:48:00 PM
Chlorine is one of the most common elements found on Earth's crust. The name comes from the Greek word for light green, which is how the gas appears in elemental form. It has 17 protons and two stable isotopes giving it a standard atomic weight of 35.45, which makes chlorine the second lightest halogen. It also has the highest electron affinity of any element making it a very strong oxidizer. This means that chlorine will readily steal electrons from other elements. In fact the vast majority of chlorine found on Earth is in the form of the chloride anion (a chlorine atom which has already stolen an extra electron), which will form ionic compounds with many cations (like metals). It is in this form that humans are most familiar with chlorine, as in ionic compound sodium chloride, which we know of as table salt. Chloride ions are important to many chemical and industrial processes including the making of usable chlorine and sodium hydroxide, and desalination and testing of potable water.
Topics: corrosion, corrosion intercept, pitting corrosion, chloride